WSF OpenID Connect Login
Step-by-step instructions to guide you through the preparation and fitting of IUS/IUD.
The summary of product characteristics should be referred to before fitting an IUS or IUD.
Key considerations when preparing for an IUS insertion:1
- Obtain informed consent and arrange for chaperone.1
- An appropriately trained assistant should be present (to monitor & support the patient and assist in an emergency).2
- Basic risk assessment includes gathering information about previous intrauterine procedures. Patients who have had previous adverse events during insertion are more likely to have them again.3
- Pulse rate and blood pressure should be documented.3
- The need for pain relief during insertion should be discussed with the patient.3
- Routine antibiotic prophylaxis is not recommended pre-insertion. However, for women at increased risk of STIs, in whom testing has not been completed, prophylactic antibiotics are advised.1
- Johnson BA. Insertion and removal of intrauterine devices, American Family Physician. January (2005). Return to content
- FSRH. Intrauterine Contraception [online] October 2015. Available from: https://www.fsrh.org/standards-and-guidance/documents/ceuguidanceintrauterinecontraception/. Last accessed March 2019. Return to content
- Mirena® Summary of Product Characteristics. Return to content
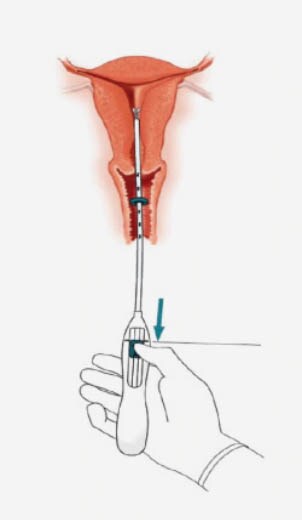
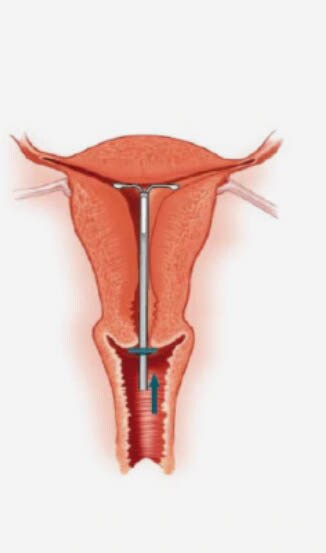
Inserting an IUS – the procedure1
- For women with symptomatic pelvic infection, postpone fitting and treat infection. Treatment should be complete before another attempt at insertion.
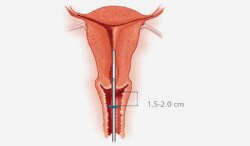
- A pelvic examination should be performed prior to inserting the device, to assess the size, shape, position and mobility of the uterus.
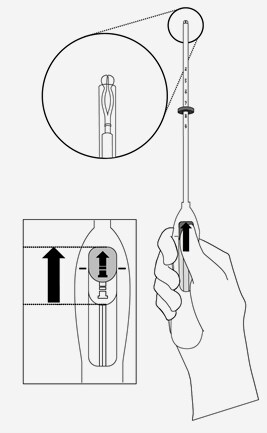
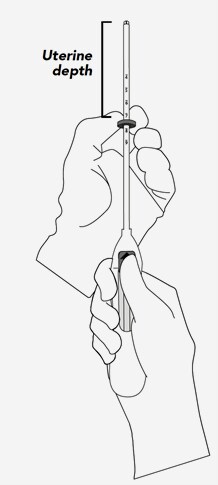
- Assessment of uterine size by sound measure.
- Forceps (tenaculum) are used to stabilise the cervix during insertion and reduce risk of perforation.
- Some fitters offer para-cervical block for the procedure.
- Many fitters use local anaesthetic gel on the cervix.
- Documentation should be made in the case notes of pre- and post-insertion counselling, the procedure, the type of device inserted, and any adverse events.
- Johnson BA. Insertion and removal of intrauterine devices, American Family Physician. January (2005). Return to content
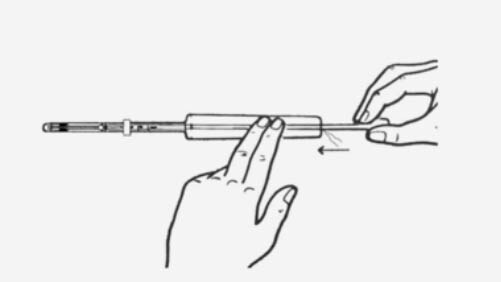
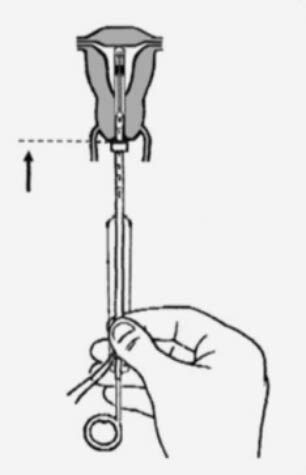
Practical aspects of fitting an IUS1
Practical aspects of fitting an IUD1
The IUD may be used by trained medical staff only. We recommend using sterile gloves to minimise the risk of contamination.
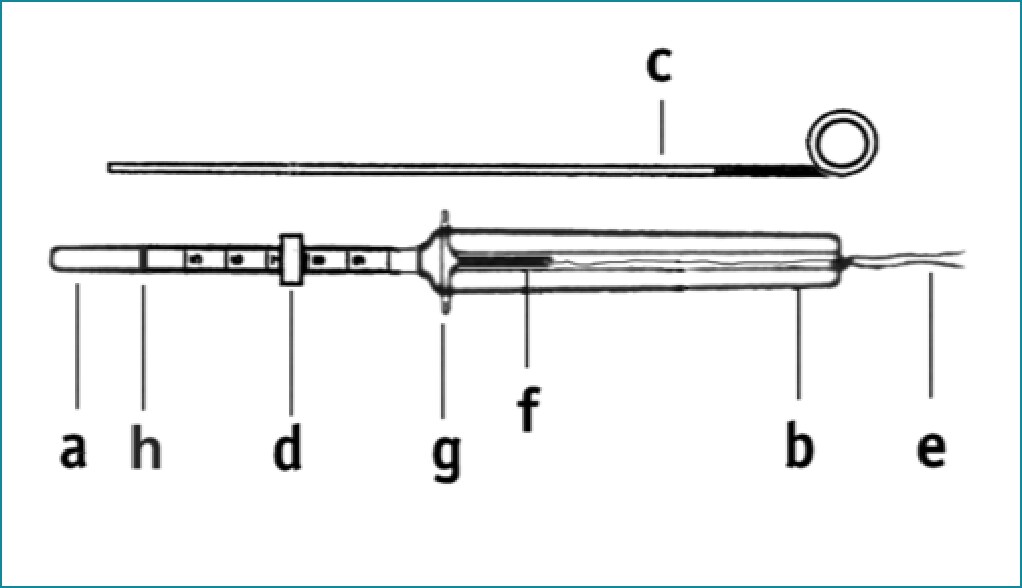
- Insertion tube
- Insertion body
- Plunger
- Blue flange
- Nylon threads
- IUD stem
- Side arms
- Blue marking
NOTE: Due to the soft and flexible insertion tube, the side arms may twist while being pushed upwards. Please adjust the sliding blue flange as described in step 3, above. The side arms of the T-Safe® CU 380A QL must not remain bent for over five minutes within the insertion tube, or they may not bend back completely and may not return to their original 90° angle.
REMOVAL: T-Safe® CU 380A QL should be replaced after ten years.
- T Safe CU 380A QL Intrauterine Contraceptive Device, Physicians Instructions, EurimPharm. Return to content
Potential problems at the time of insertion
Vasovagal collapse/cervical shock:
- Stop the procedure, lower the head and/or raise the legs. The IUD/IUS may need to be removed. An assistant shouldmonitor observations. Ensure a clear airway. ‘ABC approach’ in basic resuscitation.
- Oxygen and suction as required. Consider use of atropine IV/IM for persistent bradycardia. Consider use of adrenalineIM 1:1000 for management of anaphylaxis.
- Automated external defibrillator (AED) should be available. Arrange ambulance transfer if there is no improvement.
Syncope:
- Syncope may be experienced at insertion secondary to vagal stimulation from the cervix.1 Bradycardia is more common in nulliparous women.2
Suspected uterine perforation:1
- If perforation is suspected at the time of insertion, stop the procedure and monitor observations. Ultrasound or plain abdominal film to locate the device should be arranged as soon as possible, depending on device.
- Mirena® Product Monograph Return to content
- Farmer M, Webb A; Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003 Oct;29(4):227-31. Return to content
Post-insertion advice
Provide patients with a patient information leaflet and advice post-insertion of IUS/IUD is an important component of patient care.
Key advice to be offered includes:1
Expect cramping that usually lasts for 24-48 hours, but may last longer and resolves thereafter and usually does not persist.
Informing the patient on how to feel the threads (e.g. before the next episode of sexual intercourse and after her next menses) and advise her to seek medical advice if she is unable to feel them.
It may also be worthwhile discussing:
Bleeding
Infection
If the patient is feeling well enough to leave and is comfortable
Ensure documentation completed
A follow-up appointment with the patient for 6 weeks’ time
- Mirena® Product Monograph Return to content
Good practice points for documentation1
Detailed documentation is a key component of good practice in IUS/IUD fitting. Important points to document include:
- Faculty of Sexual and Reproductive Healthcare. Service Standards for Record Keeping in Sexual and Reproductive Healthcare Services, July 2019.
Available at: https://www.fsrh.org/standards-and-guidance/documents/fsrh-service-standards-for-record-keeping-july-2019/. Last accessed: June 2020. Return to content
Mirena® (52 mg Levonorgestrel) insertion, removal and replacement
Step-by-step video guidance for the preparation, insertion, removal and replacement of the Mirena® IUS.