WSF OpenID Connect Login
Title
Mirena® targets the endometrium directly by locally releasing LNG1
Mirena® contains
52 mg LNG1
Initial LNG release rate:
-20 μg/24 h1
Mirena® may be considered as a low dose, estrogen free option for women who want to avoid estrogen exposure1
LNG – levonorgestrel
- Mirena® Summary of Product Characteristics. Return to content
Title
Mirena® reduces menstrual blood loss by 86%, as early as 3 months after placement1
MBL reduction in women with heavy menstrual bleeding after up to 12 months of Mirena® use*1
Median MBL (mL)
Baseline
3 months
6 months
12 months
24 mL
15 mL
5 mL
-86%‡
-91%‡
-97%‡
176 mL
(≥ 80mL
MBL =
menorrhagia)
Adapted from Andersson JK and Rybo G. 1990.1
Recommend Mirena® as one of the most effective medical treatments for your patients with heavy menstrual bleeding1
HMB – heavy menstrual bleeding; MBL – menstrual blood loss
- Andersson JK and Rybo G. Obstet Gynaecol 1990;97(8):690–694. Return to content
Title
Mirena® improves hemoglobin and ferritin levels*1
Median concentration of hemoglobin and serum ferritin in menorrhagic women at baseline and after up to 3 years of Mirena® use**1
Hemoglobin levels
Mean hemoglobin
concentration (g/L)
Duration of treatment with Mirena® (months)
Baseline
3
12
36
(n=225)
(n=183)
(n=54)
(n=32)
126
132†
136†
135†
Serum ferritin levels
Mean serum ferritin
concentration (μg/L)
Duration of treatment with Mirena® (months)
Baseline
6
36
(n=212)
(n=205)
(n=32)
15.0
27.0†
56.5†
Adapted from Endrikat J et al. 2012.1
Highlight the benefits of Mirena® to your patients with heavy menstrual bleeding-related anemia
- Endrikat J, Vilos G, Muysers C et al. Arch Gynecol Obstet 2012;285(1):117–121. Return to content
Title
Mirena®: for high satisfaction in women with heavy menstrual bleeding*1
|
|
Mirena® |
Endometrial |
|---|---|---|
|
“If I had a choice, I would |
100% |
56% |
|
“I feel much better |
100% |
72% |
|
“I noticed great improvements |
89% |
56% |
Consider Mirena® for significantly higher patient satisfaction vs endometrial ablation1
- Silva-Filho AL, Pereira FAN, Souza SS et al. Contraception 2013;87(4):409–415. Return to content
Title
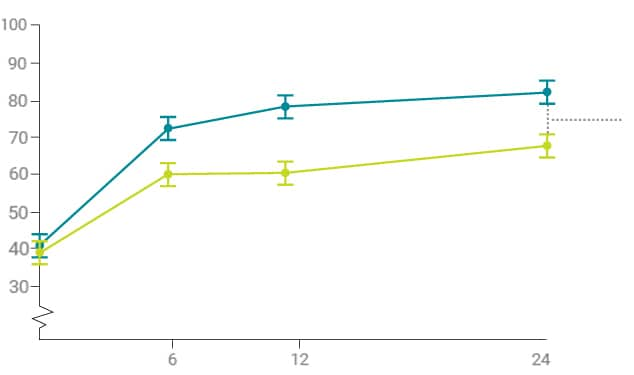
Overcoming HMB can help women improve their quality of life
Health-Related Quality
of Life (HRQoL) domains
Significantly improved
with Mirena®1
Practical difficulties
Social life
Family life
Work and daily routine
Psychological well-being
Physical health
QoL improvement with Mirena are maintained over two years
Counsel women that improving their HMB with Mirena® may lead to other quality of life improvements1
- Gupta J et al. N Engl J Med 2013;368:128–137 Return to content
Title
Mirena®: Internationally recognized as an effective treatment for women with heavy menstrual bleeding1-6
NICE
National Institute for Health
and Clinical Excellence
ACOG
American College of Obstetricians
and Gynecologists
CNGOF
French College of Obstetricians
and Gynecologists
FSRH
Faculty of Sexual and Reproductive
Healthcare.
FEBRASGO
Federacao Brasileira das Associacoes
de Ginecologia e Obstetricia
SOGC
Society of Obstetricians and
Gynaecologists of Canada
ACOG
American College
of Obstetricians
and Gynecologists
CNGOF
French College
of Obstetricians
and Gynecologists
FSRH
Faculty of Sexual and Reproductive
Healthcare.
FEBRASGO
Federacao Brasileira das Associacoes
de Ginecologia e Obstetricia
SOGC
Society of Obstetricians and
Gynaecologists of Canada
HMB – heavy menstrual bleeding
*For women with
- no identified pathology or,
- fibroids less than 3 cm in diameter, which are not causing distortion of the uterine cavity or,
- suspected or diagnosed adenomyosis.
- NICE Clinical Guideline. Heavy Menstrual Bleeding: assessment and management. March 2018. Return to content
- ACOG. Obstet Gynecol 2013;122(1):176–185. Return to content
- Marret H, Fauconnier A, Chabbert-Buffet N et al. Eur J Obstet Gynecol Reprod Biol 2010;152(2):133–137. Return to content
- FSRH Clinical Guidance. Intrauterine Contraception. September 2019. Return to content
- FEBRASGO. Guia Prático de Condutas – Tratamento do Sangramento Uterino Anormal. 2014. Return to content
- Singh S, Best C, Dunn S et al. J Obstet Gynaecol Can 2013;35(5):473–475. Return to content
Title
Counsel your patients about a long-term solution for HMB

For women who have been
fitted with Mirena® to treat HMB,
the system should be removed
or replaced in case symptoms
of HMB return.
If symptoms have not returned
after 5 years of use, continued
use of the system may be
considered. Remove or replace
after 8 years at the latest.1
Discuss with your patients how Mirena® can be a potential long-term solution to help them control their HMB1
- Mirena® Summary of Product Characteristics. Return to content
Title
Mirena®: Frequently asked questions
Below you will find answers to some commonly asked questions about the use of Mirena® for the treatment of heavy menstrual bleeding. These will support you when assessing appropriate treatment options for women in your clinical practice.
Previously, Mirena® was licensed as an IUS providing effective contraception for 6 years and for the treatment of idiopathic menorrhagia for 5 years. However, Mirena® can now remain in place for an additional 3 years if symptoms of HMB have not returned earlier and in those who wish to continue contraception with this method1
All in all, Mirena® can now remain in place to provide contraception in maximum for up to 8 years.
- Mirena® SmPC, Bayer. Return to content
Mirena® remains highly effective, providing your patients the peace of mind of continuous contraception for an additional 3 years. In an open-label extension study (N=362) there was a 0.68% cumulative pregnancy rate in Years 6–8 of Mirena® use. This was independent of patient compliance, while age and parity showed no important differences.1
- Jensen JT et al. Am J Obstet Gynecol 2022;227(6):873.e1–873.e12. Return to content
Mirena® is a long-acting reversible contraceptive method that can be particularly useful in women with heavy menstrual bleeding.1
Mirena® maintains a favorable benefit/risk profile through 8 years of use.1 For full benefit/risk information, please see the Mirena® SmPC.2
- Jensen JT et al. Am J Obstet Gynecol 2022;227(6):873.e1–873.e12. Return to content
- Mirena® SmPC, Bayer. Return to content
If your patient wishes to continue contraception, Mirena® can now remain in place for up to 8 years.
At the end of 8 years, it must be removed and replaced with a new Mirena® if the patient chooses to continue contraception with this IUS.1
- Mirena® SmPC, Bayer. Return to content
As long as symptoms of HMB remain controlled, Mirena® can stay in place for up to 8 years. In case symptoms of HMB return, Mirena® should be removed and replaced, if medically appropriate. For all women using Mirena®, the IUS must be removed after 8 years of use.1
- Mirena® SmPC, Bayer. Return to content