WSF OpenID Connect Login
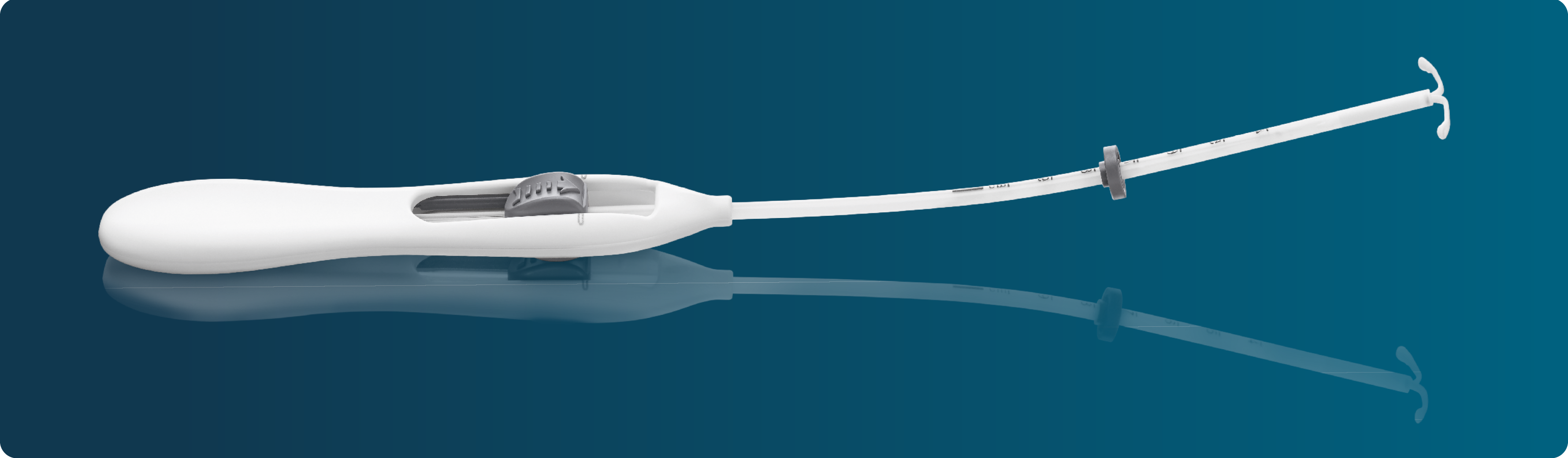
Bayer EvoInserter®: meeting your needs in intrauterine contraception
Developed in cooperation with healthcare professionals, the Bayer EvoInserter®* can streamline your IUS placement procedure, compared with earlier devices, giving you an opportunity to offer IUS to suitable women you see in your daily practice.1
Did you know? Since its introduction in May 2011, more than 49 million placements have been performed with the Bayer EvoInserter®2
The Bayer EvoInserter® allows you to focus more on what matters: taking care of your patients
IUS – intrauterine system
- The insertion device shown intends to highlight the commonalities across all Bayer IUS products and is not representative of a specific Bayer IUS brand. Return to content
- Gemzell-Danielsson K et al. Contraception 2017;96(6):426–431. Return to content
- Bayer Internal Sales Data. Return to content
Bayer EvoInserter®: simplifying the IUS placement procedure1-4
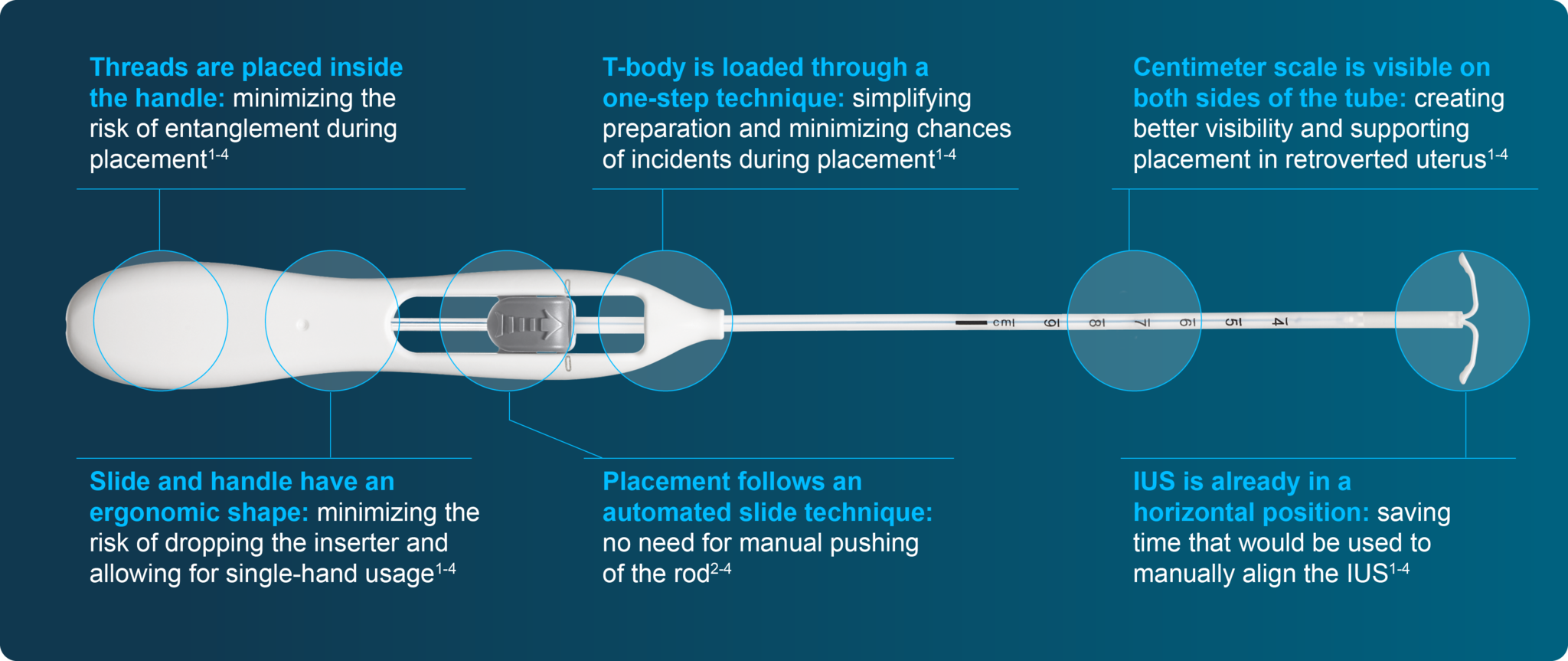
With a range of unique features, the Bayer EvoInserter® can benefit placement experience in your daily practice1
IUS – intrauterine system
- Gemzell-Danielsson K et al. Contraception 2017;96(6):426–431. Return to content
- Kyleena® Summary of Product Characteristics. Return to content
- Mirena® Insertion Instructions. Return to content
- Jaydess® Summary of Product Characteristics. Return to content
Bayer EvoInserter®: for a favorable IUS placement experience1
“The development of EvoInserter® gives healthcare professionals greater control over the placement process because of its ease of use and confidence in correct deployment” – Kristina Gemzell-Danielsson
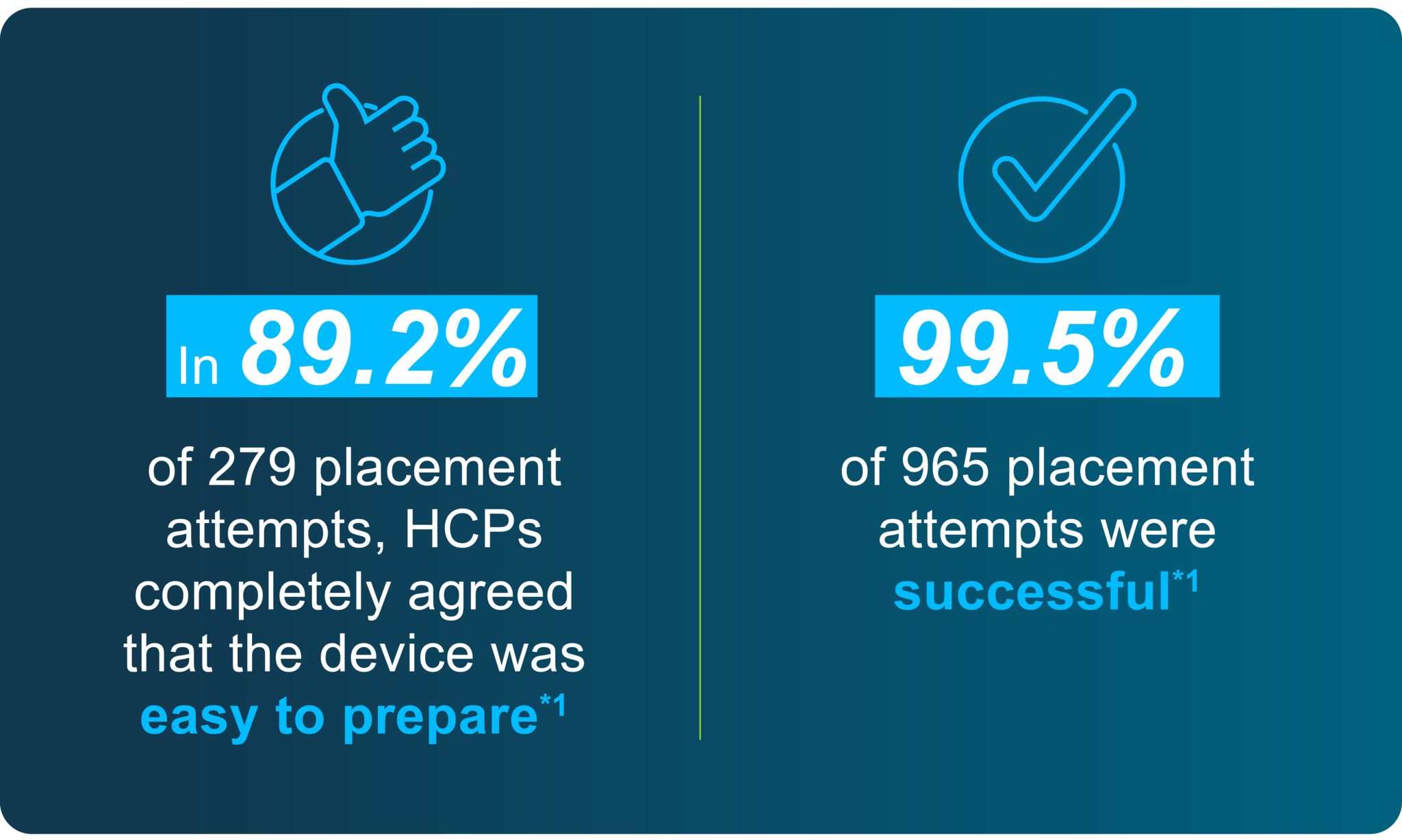
- Results from a pooled analysis using data from three previously reported Phase III studies in nulliparous (83.3%) and parous (16.7%) women aged 12–35 years (N=965). The study assessed placement success, ease of placement as reported by HCPs, pain at placement as reported by participants, and assessment of the Bayer EvoInserter® placement device by HCPs. Jaydess® was the LNG-IUS to be placed. A total of 99.5% of 965 placement attempts were successful, and HCPs completely agreed that the Bayer EvoInserter® was easy to prepare in 89.2% of 279 placement attempts.1 Return to content
Jaydess® insertions may be considered easier and less painful with the smaller T-body and the narrower placement tube compared to other IUS.
HCP – healthcare professional; IUS – intrauterine system; LNG – levonorgestrel
- Gemzell-Danielsson K et al. Contraception 2017;96(6):426–431 and Supplementary Appendix. Return to content
Bayer EvoInserter®: easy placements, no matter women’s parity1
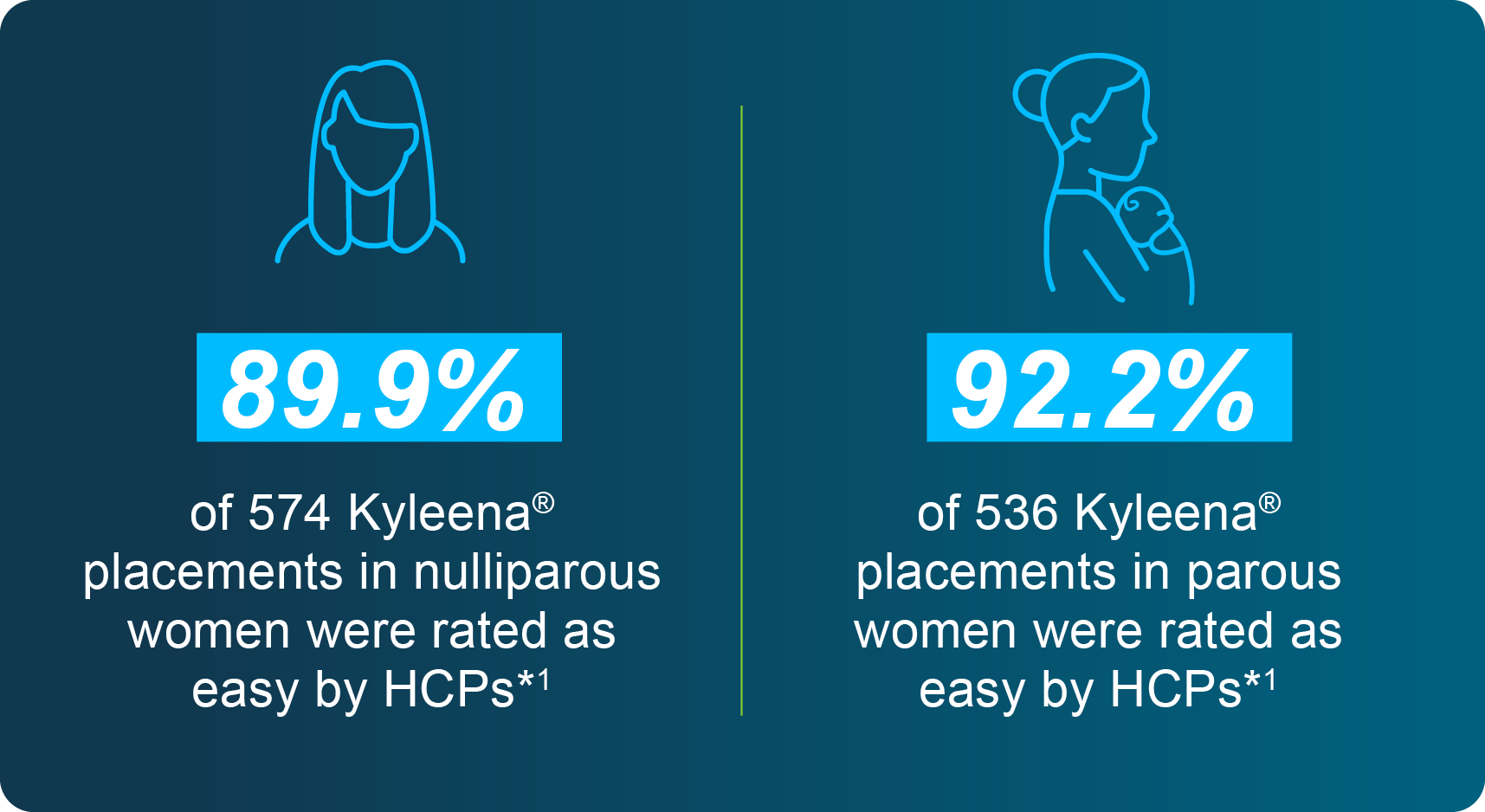
Discuss with your patients how the majority of placements with the Bayer EvoInserter® are simple, no matter their parity1
- Results from a baseline analysis of the Kyleena® Satisfaction Study (KYSS) data, a real-world study
conducted to evaluate demographics, ease of placement assessed by investigators, pain at placement
rated by women, additional interventions for placement, and adverse events. A total of 1,110 women
(574 nulliparous and 536 parous) from seven countries were included in this analysis. Kyleena® was the
LNG-IUS to be placed. A total of 91% of the 1,110 placement with the Bayer EvoInserter® were rated as
easy by HCPs, regardless of women’s parity.1
Return to content
Kyleena® insertions may be considered easier and less painful with the smaller T-body and the narrower placement tube compared to other IUS.1
HCP – healthcare professional; IUS – intrauterine system; LNG – levonorgestrel
- Beckert V et al. Eur J Contracept Reprod Health Care 2020;25(3):182–189. Return to content
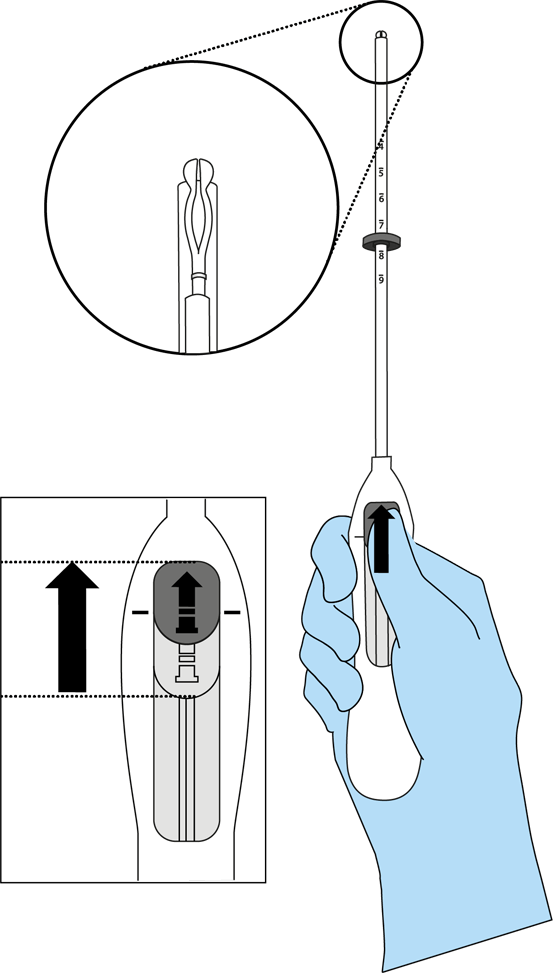
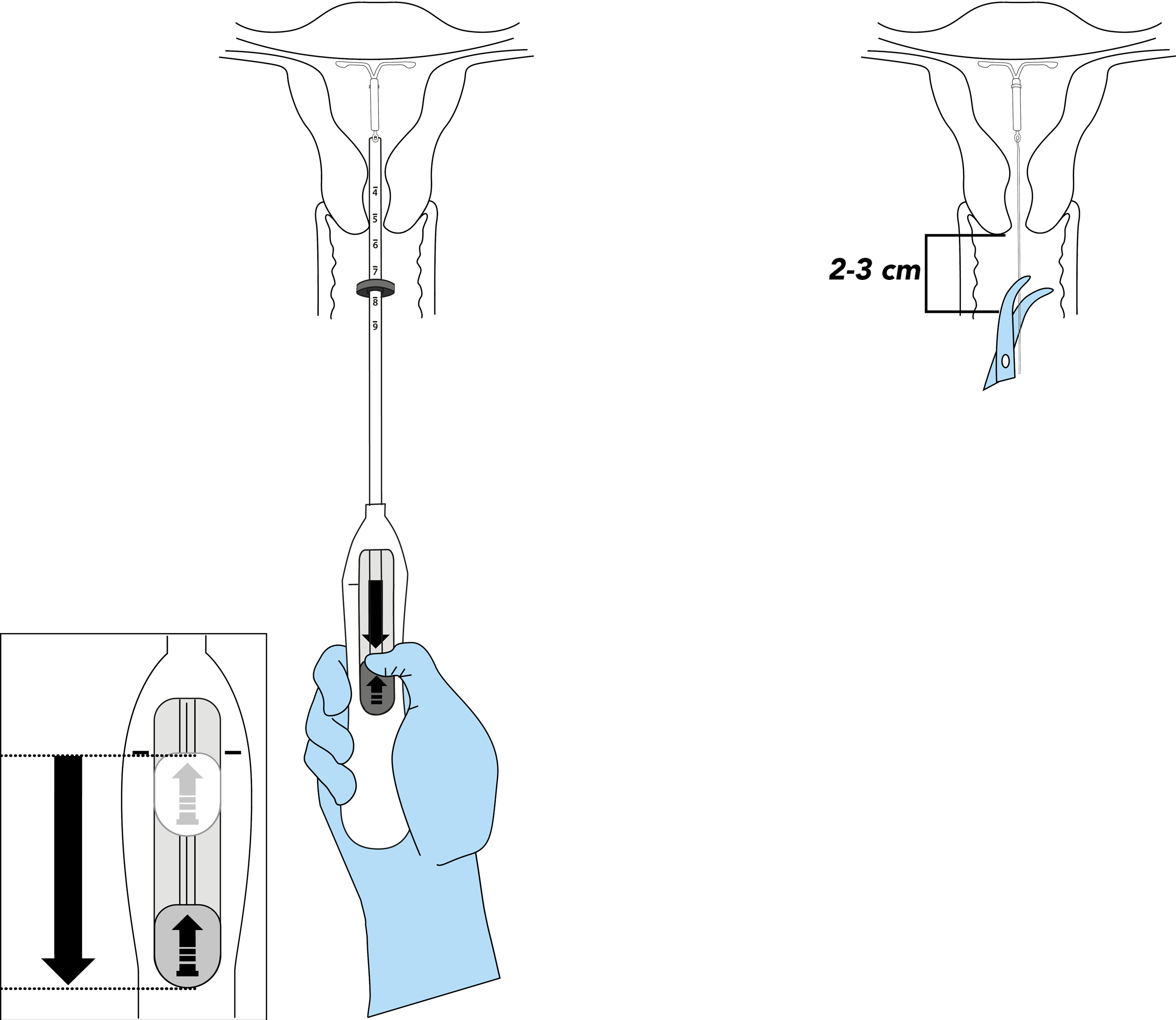
Bayer EvoInserter®: how to prepare for IUS placement
Preparations for IUS placement take around 5–10 minutes, with the actual placement only taking a few minutes
The preparation for insertion:1-4
- Examine the patient to establish the size and position of the uterus, in order to detect any signs of acute genital infections or other contraindications for the insertion of Mirena and to exclude pregnancy.
- Insert a speculum, visualize the cervix, and then thoroughly cleanse the cervix and vagina with a suitable antiseptic solution.
- Employ an assistant as necessary.
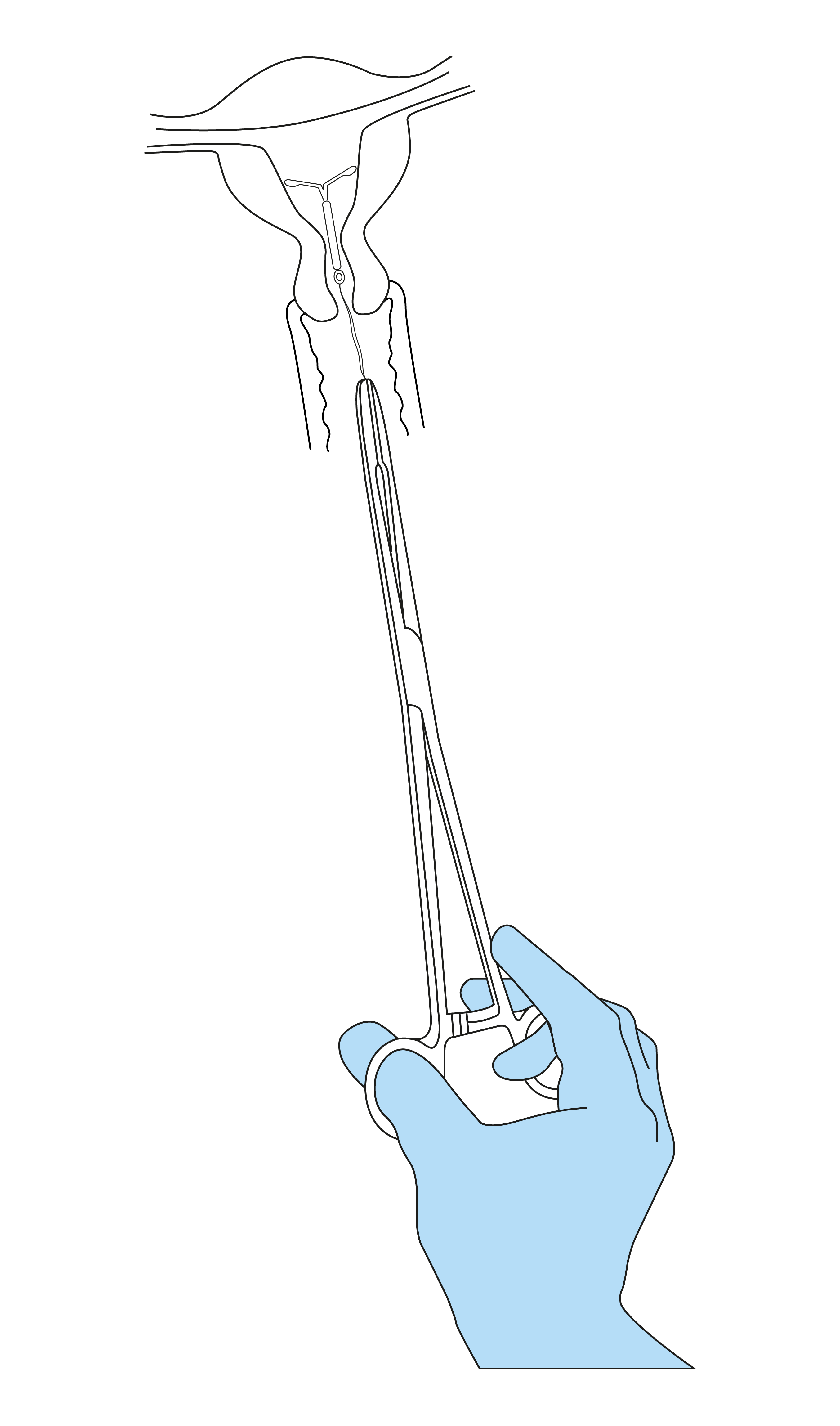
- Grasp the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip of the cervix. Gentle traction on the forceps can be applied to straighten the cervical canal. The forceps should remain in position and gentle counter traction on the cervix should be maintained throughout the insertion procedure.
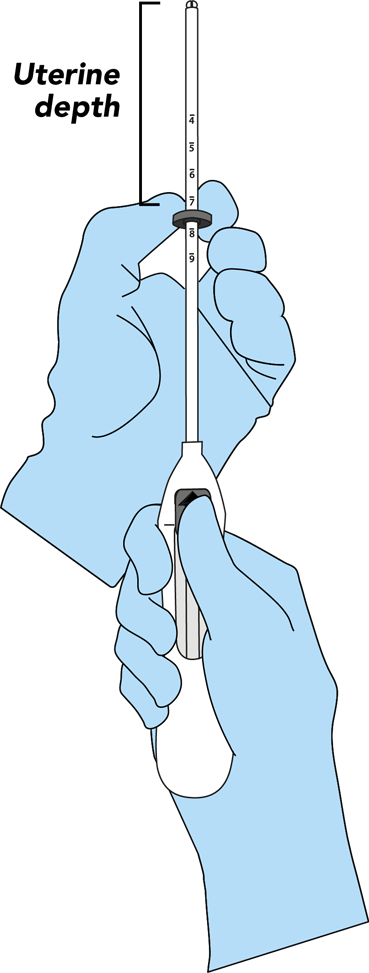
- Advance a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to exclude any evidence of intrauterine abnormalities (e.g., septum, submucous fibroids) or a previously inserted intrauterine contraceptive which has not been removed. If difficulty is encountered, consider dilatation of the canal. If cervical dilatation is required, consider using analgesics and/or a paracervical block.
Prepare for IUS placement in minutes, with the Bayer EvoInserter®
IUS – intrauterine system
- Mirena® Insertion Instructions. Return to content
- Mirena® Summary of Product Characteristics. Return to content
- Kyleena® Summary of Product Characteristics. Return to content
- Jaydess® Summary of Product Characteristics. Return to content
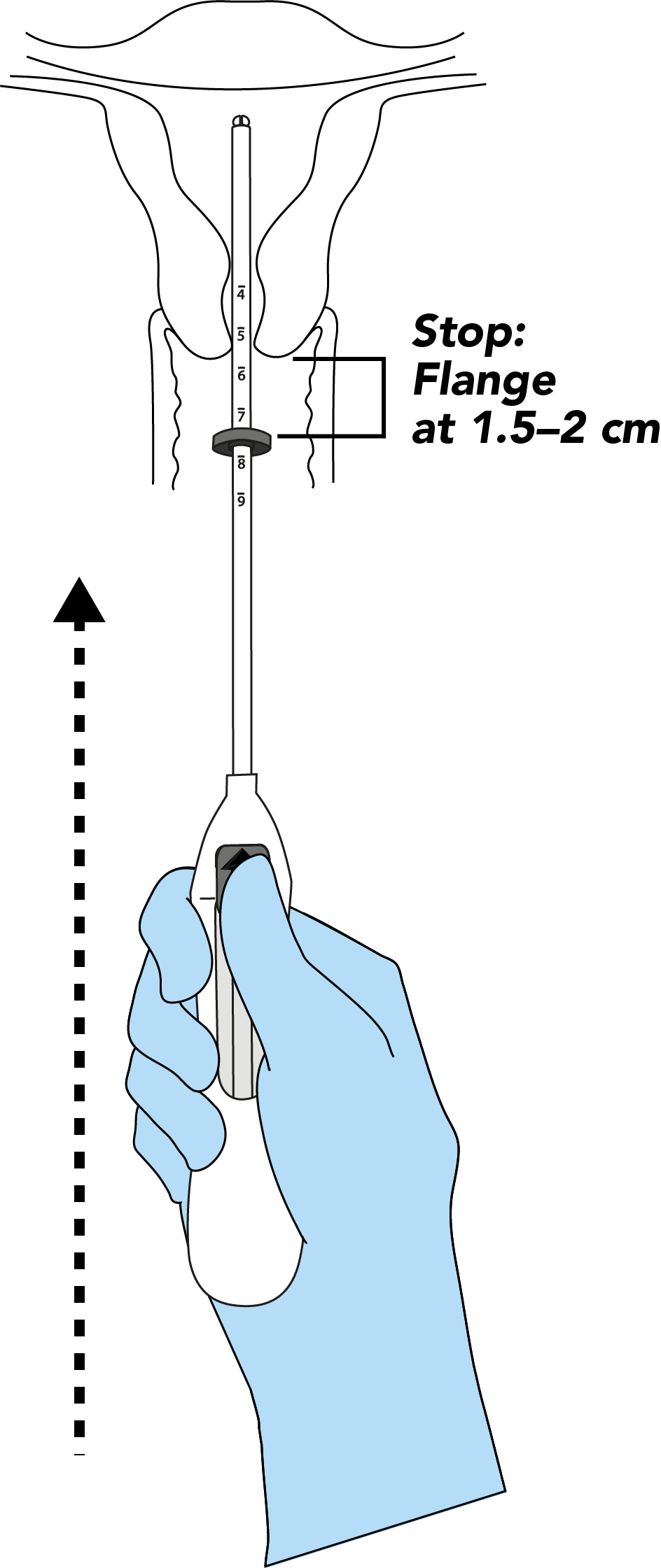
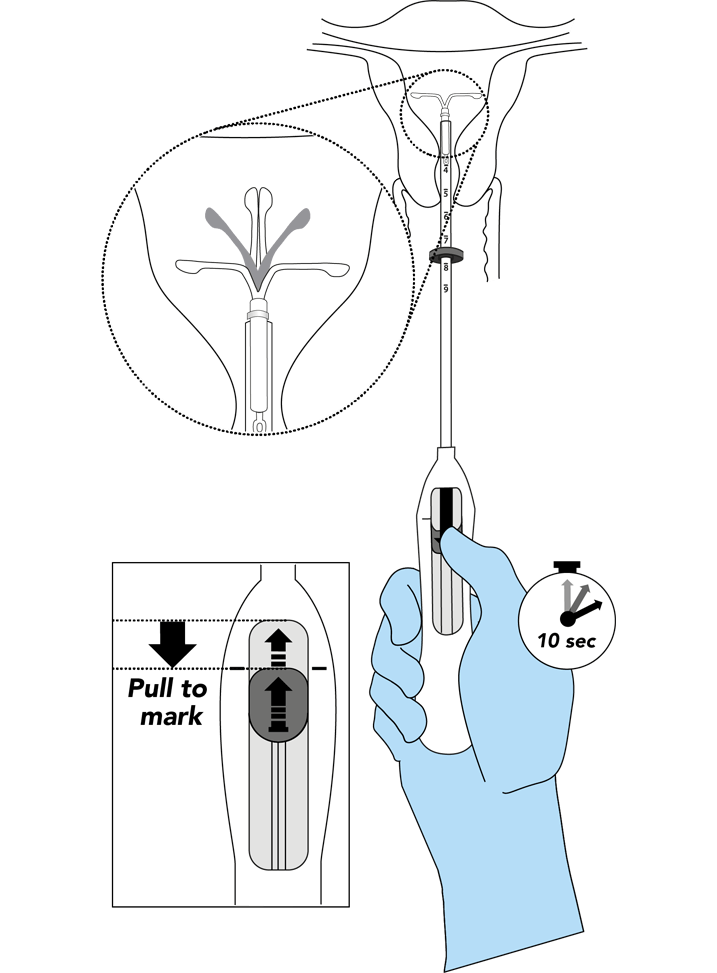
Bayer EvoInserter®: IUS placement in seven simple steps1-3
Consider how the Bayer EvoInserter® can prompt the use of IUS in your clinical practice
IUS – intrauterine system
- Kyleena® Summary of Product Characteristics. Return to content
- Mirena® Insertion Instructions. Return to content
- Jaydess® Summary of Product Characteristics. Return to content
Bayer EvoInserter®: IUS removal / replacement1-3
Kyleena® needs to be replaced after five years of continuous use.1
For Mirena®, Please adapt to reflect local market labels.
Jaydess® needs to be replaced after three years of continuous use.*3
Removal should be carried out within seven days of the onset of menstruation. To remove, gently pull on the IUS threads with forceps.1-3
- Removal/replacement instructions, including limit time for continuous use, may vary between countries and indications. Return to content
IUS – intrauterine system
- Kyleena® Summary of Product Characteristics. Return to content
- Mirena® Summary of Product Characteristics. Return to content
- Jaydess® Summary of Product Characteristics. Return to content